Published Apr 21, 2025 | 8:00 AM ⚊ Updated Apr 21, 2025 | 8:00 AM
Synopsis: A batch of Telma 40, a hypertension drug, has been flagged as spurious by CDSCO in its March 2025 alert. Batch 5240226 failed Indian Pharmacopoeia standards. The listed manufacturer denied producing it, raising concerns of counterfeit. Telma 40 treats high blood pressure and heart failure. An investigation is currently underway
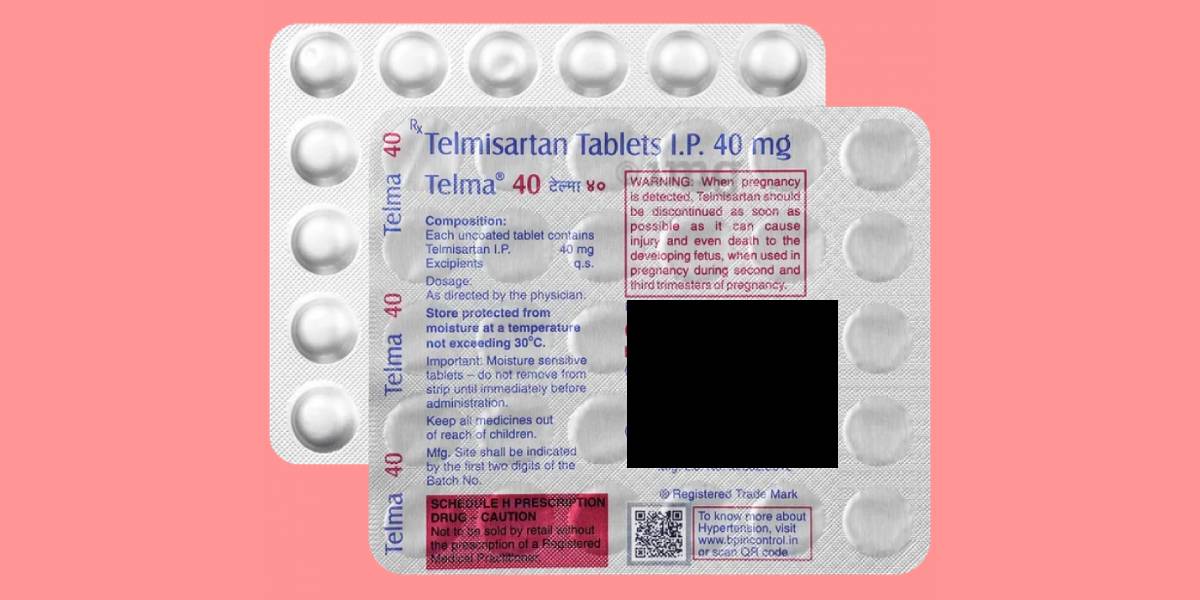
A batch of Telma 40, a widely prescribed drug for hypertension, has been found to be spurious by the Central Drugs Standard Control Organization (CDSCO) in its latest quality alert for March 2025. The affected batch number 5240226, manufactured by a reputed Indian pharmaceutical company, was flagged during routine testing.
According to the CDSCO, the batch “does not conform to I.P.”, meaning it fails to meet the standards outlined in the Indian Pharmacopoeia (I.P.). Spurious drugs are considered fake or counterfeit; they often contain incorrect, contaminated, or no active ingredients, posing serious health risks to patients.
“The product is purported to be spurious; however, the same is subject to the outcome of the investigation,” stated the CDSCO.
Telma 40 is used to treat high blood pressure and heart failure. It helps prevent heart attacks and strokes.
In a significant development, the actual manufacturer, as stated on the drug’s label, has denied producing the impugned batch. This further confirms the likelihood of the product being spurious, the CDSCO noted.
This is not an isolated incident. Just last month, Telma H, another antihypertensive drug from the same manufacturer, was also declared spurious. Telma H is typically prescribed for patients with high blood pressure and fluid retention, often associated with heart failure.
In January, yet another product — Telma AM, also from the same drug category — was found to be Not of Standard Quality (NSQ). While Telma AM is used to improve blood circulation without affecting kidney function, it failed to meet the required pharmacopoeial.
From eye ointments used to treat conjunctivitis to tablets prescribed for anxiety, a wide range of medicines — 70 batches in total — have been flagged as Not of Standard Quality (NSQ) by India’s drug regulatory authority in March 2025. The monthly alert released by the CDSCO highlights continued concerns over the quality of drugs circulating in the Indian market.
Among the flagged products is Chloramphenirol Eye Ointment, typically used for treating bacterial eye infections, which failed the assay test for active ingredient. Meanwhile, Alprazolam tablets, a commonly prescribed anti-anxiety medication, were found substandard due to dissolution failure, raising red flags about their therapeutic effectiveness.
The list spans several therapeutic categories including antibiotics, vitamins, gastrointestinal drugs, injectables, and cardiovascular medicines. Other commonly consumed medicines such as Pantoprazole, Domperidone, Cholecalciferol (Vitamin D3), Iron Sucrose Injections, and Calcium-Vitamin D3 supplements also featured prominently in the alert.
Gidsha Pharmaceuticals, a Gujarat-based firm, topped the list with eight separate batches of its calcium and Vitamin D3 tablets (marketed as CALXIA-500) failing dissolution tests. Affy Parenterals, Martin and Brown Biosciences, Zee Laboratories, and Hindustan Antibiotics Ltd. were also among the top repeat offenders.
The substandard findings were based on routine sampling and testing by government laboratories including the Central Drug Laboratory (CDL) Kolkata, and Regional Drug Testing Labs (RDTLs) in Guwahati, Mumbai, and Chandigarh.
According to the CDSCO, drugs are classified as NSQ when they fail to meet parameters such as active ingredient content, dissolution, microbial contamination, sterility, or assay. While not all NSQ drugs are necessarily harmful, many can be therapeutically ineffective or pose health risks, especially when consumed by vulnerable populations.
A routine quality surveillance drive by India’s State Drug Laboratories has flagged 61 drug and cosmetic products as Not of Standard Quality (NSQ) for the month of March 2025, exposing alarming lapses in the safety and quality of widely used medicines and personal care items.
Among the most concerning findings was a paracetamol syrup for children, which was flagged as misbranded for failing to disclose critical labeling information. Equally disturbing was the discovery of high levels of mercury in several cosmetic products, including lipsticks and skin creams — substances that are strictly regulated due to their potential health hazards.
The Central Drugs Standard Control Organization (CDSCO) received this data from seven state laboratories, with the Drugs Testing Laboratory in Thiruvananthapuram (Kerala) accounting for the highest number of NSQ reports (28), followed by Telangana (18), West Bengal (5), Jammu & Kashmir (4), DTL Baddi, Himachal Pradesh (3), CDL Kolkata (2), and Uttarakhand (1).
The report lists 52 pharmaceutical products that failed on various counts, including, Paracetamol IP (Syrup and Tablets) – A staple antipyretic and pain reliever used across all age groups, with batches failing dissolution and labeling standards. Albendazole Tablets IP 400 mg, a common deworming agent, with nine separate batches failing the dissolution test, raising questions about bioavailability. Glimepiride Tablets (Anti-diabetic), Telmisartan Combinations (Antihypertensives), and Mefenamic Acid formulations (Pain relief) were also among those declared substandard.
Injectables like Vitamin B12 and Oxytetracycline (Veterinary) were reported to have failed sterility and description requirements.
Equally alarming is the presence of toxic heavy metals like mercury in cosmetic products, some of which are imported and widely marketed:
Lipsticks, skin-lightening creams, and facial scrubs were found to contain mercury well beyond permissible limits, in violation of the Cosmetic Rules, 2020. Products from brands like Faiza Beauty Cream, Blue Heaven, Goree, and Top UP Lipstick were among those flagged. Baby shampoos and face washes also failed microbial and labeling standards, posing a direct risk to children and individuals with sensitive skin.
(Edited by Ananya Rao)

