
Representational image of a cough syrup. Kerala has stopped supply and sale of all drugs by Maiden Pharmaceuticals. (Creative Commons)
The Kerala government has proactively ordered a complete halt to the supply and sale of all the drugs made by Haryana-based Maiden Pharmaceuticals Ltd, the company whose cold-and-cough syrups have been linked by WHO to the deaths of 66 children in the West African nation of Gambia.
At a time the Union Health Ministry is yet to initiate any action against Maiden Pharma, Kerala has also directed its drug inspectors to collect samples of every drug and syrup manufactured by the company and send them for testing immediately.
Confirming this to South First, Kerala State Drug Controller, PM Jayan said: “A blanket order has been issued instructing all drug inspectors to immediately stop the supply of any drug manufactured by Maiden Pharmaceuticals and to take random samples for quality checking. Only if the samples get clearance in the quality-control test can they allow distribution of drugs by this company in the market.”
The State Drug Control Department has also ordered its drug inspectors to check with paediatricians about commonly prescribed cough-and-cold syrups and has asked them to collect samples of even those syrups to rule out any possibility of the presence of diethylene glycol and ethylene glycol — the toxins that caused deaths of the children in the Gambia.
“The presence of diethylene glycol and ethylene glycol can be extremely dangerous. It is better to rule out their presence. As a precautionary measure, random samples of commonly prescribed syrups from other companies will also be collected and sent for quality checks,” Jayan said.
“Maiden Pharmaceutical’s cough syrups required polyethylene glycol; similarly, what if other drug companies have also used it? This needs to be checked,” he added.
In 2021 and 2022, Kerala’s drug authorities have found Maiden Pharmaceutical’s products to be of substandard quality as many as four times. The authorities are preparing the paperwork to prosecute the company for the supply of substandard quality calcium tablet Maical D.
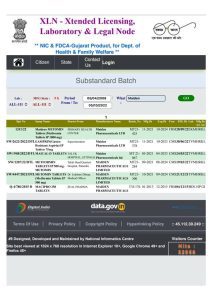
List of drugs to by Maiden Pharmaceuticals against which Kerala government had raised a red flag for ‘substandard’ quality. (https://xlnindia.gov.in/)
“We are preparing the necessary documents and the company will be prosecuted here. Red flags have been raised on this drug, along with three other drugs; a departmental action is being initiated under the Drugs and Cosmetics Act. We will prepare the charge sheet too,” Jayan said.
A gastro-resistant aspirin tablet (Easiprin IP 75 mg tablet), calcium tablet (Maical D), two diabetic tablets (Metformin IP 500mg and Metomin) — all four manufactured by the Maiden Pharmaceuticals — were removed from all pharmacies of Kerala earlier.

Representational picture of Metaformin tablets manufactured by the Maiden Pharmaceuticals Ltd, a Haryana-based company whose four cough syrups are suspected to be the reason for the deaths of 66 children in the Gambia. (Wikimedia Commons)
Speaking to South First, a senior drug inspector said it was “surprising for us that despite alerting and red-flagging of these drugs from the company on the eXtended Licensing, Laboratory and Legal Node (XLN) database that is maintained by the Government of India, no action has been taken against the company,”
However, Jayan said that the state, in it’s routine quality checks, had picked six drugs supplied by Maiden Pharmaceuticals. Of the six, four were found to be of substandard quality and hence were removed from the shelves.
“We picked Metformin, type-2 diabetes tablets from taluk headquarter hospital in Palakkad in March 2022 and from a primary health care centre in Ernakulam in September 2022. Both sets failed the dissolution test. The test showed that the drug wasn’t able to dissolve properly in a given amount of time, thus failing to release the necessary medicine into the body,” he said.
Assistant Drug Controller Sasi PK told South First that, as part of a routine check, the Metformin sample was picked up in Palakkad district and sent to the lab. After it was found to be substandard, “we immediately sent back 1,900 tablets at the centre to the company. We also instructed all drug inspectors to stop supply of this medicine and take them off the shelves”.
The drug Easiprin, picked up from a hospital in Kannur in June 2022, failed the salicylic-acid test, The drug contained too much aspirin, which could lead to poisoning and cause ringing in the ears, drowsiness and even acute dehydration.
In case of Maical-D tablet, Jayan said that the tablet used to treat vitamin D and calcium deficiency had failed in the tests too.
The @WHO issued a Medical Product Alert a couple of days back about DEG poisoning and death of over 60 children in Gambia. https://t.co/vPgriIIJuO
This thread documents the persistent, callous, unaccountable and nonchalant attitude of every #publichealth
1/n pic.twitter.com/IrCMuqKUlm
— Dinesh S. Thakur (@d_s_thakur) October 6, 2022

Mar 22, 2024

Mar 14, 2024

Mar 14, 2024

Mar 13, 2024

Mar 12, 2024

Mar 11, 2024