Published Sep 21, 2022 | 7:05 PM ⚊ Updated Sep 23, 2022 | 5:39 PM

As vaccine development is going forward with multiple candidates in the race, the evolution of the virus itself raises questions about their efficacy (Creative Commons)
The Hyderabad-based Indian Immunological Limited (IIL) recently became the fourth company to receive permission for a Phase-1 trial of the first dengue vaccine in the country — but most scientists South First spoke to were sceptical of the outcome of such trials or advised caution.
The reason: Dengue is a peculiar disease.
As the journal Science reported, the first dengue infection is rarely fatal, “but a second one with a different virus type can lead to much more serious disease, because of what is called antibody-dependent enhancement (ADE), in which the immune response to the first virus amplifies the effect of the second type”.
In India, 35 states and Union territories, except Lakshadweep, have reported dengue cases in the last two decades, and its incidence is increasing in frequency. It is one of the most extensively spread arboviral diseases and is transmitted by Aedes mosquitoes.
All four dengue serotypes have been reported in India (Creative Commons)
All four dengue serotypes (DENV-I to DENV-IV) have been reported in India. If a person is infected with dengue for the second time with the strains DENV-II, III, and IV, it is likely to result in severe forms like dengue hemorrhagic fever (DHF) and dengue shock syndrome. This is one of the main concerns for the scientists.
Clinical studies have shown that dengue vaccination may mimic a primary infection (i.e., exposure to dengue for the first time). Vaccination can thus promote ADE in someone who has not had a primary infection before vaccination but gets infected for the first time later.
“In dengue, generally, the first infection (with any one of the dengue virus serotypes I, II, III, or IV) goes off without causing much disease, and helps develop immunity in the patient. But if the same patient encounters secondary infection [exposure to dengue for the second time] with another serotype, it may cause severe dengue,” Dr Mayilsamy Muniaraj, scientist at ICMR-Vector Control Research Centre (VCRC) in Madurai, told South First.
He added that there are chances the same thing may happen with inoculations too because vaccines are inactivated or killed forms of the viruses. “A dengue vaccine trial conducted in the Philippines miserably failed, killing many children,” said Dr Muniaraj.
As vaccine development is going forward with multiple candidates in the race, the evolution of the virus itself raises questions about their efficacy.
“These vaccines are based on the old dengue isolates from outside South Asia. In the absence of efficacy studies in India, it remains unclear whether they will induce optimal levels of neutralising antibodies against the dengue viruses circulating here,” said a recent study done by scientists of the National Centre for Biological Sciences (NCBS) and Indian Institute of Science (IISc), both located in Bangalore.
They added that apart from not providing sufficient protection against dengue, some vaccine candidates can even lead to enhanced disease upon subsequent infection.
“All the strains used by tetravalent vaccines are based on the strains isolated between 1964 and 1988. As the prevalent Indian dengue viruses are evolving under high population seropositivity, they might be diverging antigenically from the vaccine strains as well,” the study adds.
Dr Arun Sankaradoss, a postdoctoral fellow in the dengue vaccine development programme at NCBS and also the co-author of the above paper, told South First that to design a dengue vaccine candidate, we primarily need to understand the contemporary dengue serotype and their genotype diversity across geographical locations.
“Secondly, the dengue vaccine candidate’s tetravalent (all four serotypes) neutralising antibody potential and protective efficacy need to be investigated” thoroughly, said Dr Arun.
“An ideal dengue vaccine candidate needs to induce both humoral and cellular immune responses. Additionally, ADE concerns also need to be addressed in the vaccine design,” he added.
In the Philippines, around 750 people die per year because of dengue infection. In April 2016, the country began a dengue vaccination programme for nine-year-old children.
The programme involved around 8.75 lakh kids who received at least one dose of the Dengvaxia vaccine developed by Sanofi Pasteur, a France-based pharma company, while four lakh received two doses, and 3.5 lakh received all three doses.
In December 2017, the inoculation programme was terminated by the government after 12 confirmed deaths since the vaccination. As of September 2018, 19 dengue deaths of vaccinated children had been confirmed.
The Dengvaxia deaths also led to Covid-19 vaccine hesitancy in the Philippines in 2021.

Representative image of vaccine administration. The dengue vaccine fiasco in the Philippines led to hesitancy over Covid-19 jabs (Wikimedia Commons)
“Once already exposed to the dengue virus, your body will be immunised already; when you give the vaccine again, your immune system will over-react, which may cause an adverse event. This is what happened in the Philippines. We may be genetically different from people in the Philippines, but given that there are doubts, it should not be implemented immediately,” a scientist from ICMR-National Institute of Epidemiology (NIE) in Chennai, who did not want to be named, told South First.
Dr Arun Sankaradoss said that the phase 3 clinical trial data and subsequent follow-up studies of Dengvaxia revealed that this vaccine efficacy differed by age, serostatus, and serotype.
“This vaccine performed poorly against dengue serotype II and sensitised dengue-naive recipients to severe dengue upon subsequent natural infection. It also provided only low protective efficacy in younger children,” said Dr Arun.
He further added that there are several findings and speculations about the adverse events induced by this vaccine candidate. These include:
Serotype diversity of dengue viruses could be one of the reasons for the imbalanced immune response induced by Dengvaxia. This vaccine elicits the highest vaccine efficacy against DENV-IV, followed by DENV-III and DENV-I, but it performs poorly against DENV-II.
It has also been speculated that Dengvaxia did not elicit the right dengue T-cell (part of the immune system and develops from stem cells in the bone marrow) responses since it was developed in the Yellow Fever-17D backbone (vaccine), which lacks dengue non-structural proteins.
However, scientists are certain that the vaccine candidate will give a boost in controlling the virus.
“Viruses will evolve, but not all. The frequency of mutations may be very low in some (e.g., smallpox virus) and very high in others (influenza virus). In the dengue virus, the mutation frequency is also low. Vaccines will be effective for those that generally have a low mutation rate,” said Dr Muniaraj.
“The better way to prove the efficacy of the vaccine is a clinical trial. The vaccines that have been approved in the US cannot be given in India. It should be clinically proved in India first,” said the ICMR-NIE scientist.
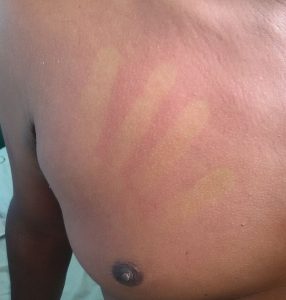
Rashes due to dengue fever (Wikimedia Commons)
According to the National Centre for Vector Borne Diseases Control, 1,93,245 cases of dengue and 346 deaths were reported across the country last year.
A study found the sero-prevalence of dengue virus infection in India at 48.7 percent, while it was as high as 76.9 percent in the southern states. DENV-I and II were the predominant serotypes in the northern and eastern regions, while it was DENV-III, II, and I in the western and southern regions.
“The nodal approval agencies in India, the Drugs Controller General of India (DCGI) and Central Drugs Standard Control Organisation (CDSCO), have to check the clinical trial where the benefit and risk ratio should be analysed carefully. And upon that, the vaccine should be given approval in India,” the ICMR-NIE scientist noted.
There are three dengue vaccines that have already been in clinical trials in different parts of India.
Panacea Biotec, Sanofi India, and Takeda Pharmaceutical have got permission for clinical trials in India. These three companies are also doing trials in other parts of the world.
Panacea Biotec Limited has completed Phase-1 and Phase-2 trials in India. The results of this trial showed that 81.9 percent achieved seroconversion for DENV-I, 77.8 percent for DENV-II, 81.9 percent for DENV-III, and 79.2 percent for DENV-IV.
Sanofi India’s Dengvaxia — the same vaccine that caused deaths in the Philippines — has already been approved in the US. The firm has started conducting trials in India.
This vaccine is recommended by the World Health Organisation (WHO) only for adults with a history of prior dengue virus infection. A safe and efficacious dengue vaccine continues to be sought globally.
Takeda Pharmaceutical, a Japanese company, has started clinical trials of its tetravalent dengue vaccine candidate, TAK003, in India. It has got permission to do clinical trials on 480 people across the country. Takeda has got approval for its dengue fever vaccine in Indonesia.
GlaxoSmithKline (GSK), a British pharmaceutical company, Butantan, a group of Brazilian medical institutions, and Merck, a US-based pharma company, are developing dengue vaccines in clinical trials in different parts of the world.
“To my knowledge, currently, adverse effects data is available only for Dengvaxia. TAK003 is another leading vaccine candidate. The TAK003 vaccine, like Dengvaxia, elicits an imbalanced immune response across serotypes, with it being most effective against Dengue serotype-II while Dengvaxia was most effective against Dengue serotype-IV,” said Dr Arun.
Considering ADE issues associated with dengue infection, recent Indian vaccine efforts, including the Sankaradoss group, are focusing on targeting the envelope domain III (EDIII— dock to body receptors to gain entry into cells) of dengue viruses for vaccine design.
EDIII plays a significant role in defining antigenicity — the ability to specifically combine with the final products of the immune response — and is the primary target of the neutralising antibodies. Antibodies directed against EDIII are more likely to circumvent the reinfection problem.
“In addition to EDIII … non-structural protein 1 (NS1) has also emerged as an attractive vaccine target. NS1, a highly conserved protein among flaviviruses [like dengue], is involved in virus replication and immune evasion. NS1 has been demonstrated to trigger T-cell responses in both experimental animals and humans. Currently, we are focusing on the optimisation of this vaccine construct and non-human primate studies,” Dr Arun added.

